Density
Absolute Density (ρ)
Absolute density is an intensive property that measures the concentration of mass per unit volume of a substance.
Fundamental Formula
where:
- : Absolute density [kg/m³ (SI)]
- : Mass of the substance [g, kg]
- : Volume occupied [cm³, m³, L]
Common Units by State of Matter
- Solids and liquids: g/cm³, g/mL, kg/L, lb/ft³
- Gases: g/L, kg/m³ (at standard conditions)
Useful Conversion Factors
Density of Some Substances at One Atmosphere of Pressure and 20°C
Densities measured at standard conditions (P = 1 atmosphere and T = 0°C)
Solids
| Substance | S.I. (kg/m³) | Common (g/cm³) |
|---|---|---|
| Cork | 240 | 0.24 |
| Wood | 500 | 0.50 |
| Paper | 700 | 0.70 |
| NaCl | 2160 | 2.16 |
| Cu | 8920 | 8.92 |
| Au | 19300 | 19.3 |
| Os | 22400 | 22.4 |
| Lead | 11300 | 11.3 |
| Glass | 2600 | 2.6 |
| Brick (average) | 1900 | 1.9 |
| Hard Rubber | 1200 | 1.2 |
Liquids
| Substance | S.I. (kg/m³) | Common (g/cm³) |
|---|---|---|
| Chloroform | 1500 | 1.50 |
| Ethyl Alcohol | 780 | 0.78 |
| Oil | 800 | 0.80 |
| Seawater | 1040 | 1.04 |
| Milk | 1030 | 1.03 |
| Blood | 1060 | 1.06 |
| Bromine | 3120 | 3.12 |
| Mercury | 13600 | 13.6 |
| Glycerin | 1260 | 1.26 |
| Sulfuric Acid | 1840 | 1.84 |
| Gasoline (approx.) | 670 | 0.67 |
Gases
| Substance | S.I. (kg/m³) | Common (g/L) |
|---|---|---|
| H₂ | 0.09 | 0.09 |
| Air | 1.29 | 1.29 |
| O₂ | 1.43 | 1.43 |
| CO₂ | 1.98 | 1.98 |
Density of Mixtures (ρₘ)
For homogeneous or heterogeneous mixtures, the density is calculated as:
General Formula
Special Cases
1. Components with Equal Volumes
When each component occupies the same volume:
Example: Mixture of 3 liquids with equal volumes:
2. Components with Equal Masses
When each component has the same mass:
Example: Mixture of 2 substances with equal masses:
Relative Density (Specific Gravity)
Relative density (ρᵣ or SG) is a dimensionless quantity that compares the density of a substance to a standard reference.
Formula
Reference Substances
| State | Reference Substance | Conditions | Reference Density |
|---|---|---|---|
| Solids and liquids | Pure water | 4°C and 1 atm | |
| Gases | Dry air | S.T.P. (0°C, 1 atm) |
Interpretation
- SG < 1: The substance is less dense than the reference (floats)
- SG = 1: Density equal to the reference
- SG > 1: The substance is denser than the reference (sinks)
Specific Weight (γ)
An intensive property that relates the weight of a substance to its volume.
Definition and Formula
Units
- International System (SI): N/m³
- Technical System: kgf/m³
Relationship with Density
Where (standard acceleration of gravity)
Relative Specific Weight (γᵣ)
Relationship between the specific weight of a substance and that of the reference substance.
Important Property
Proof:
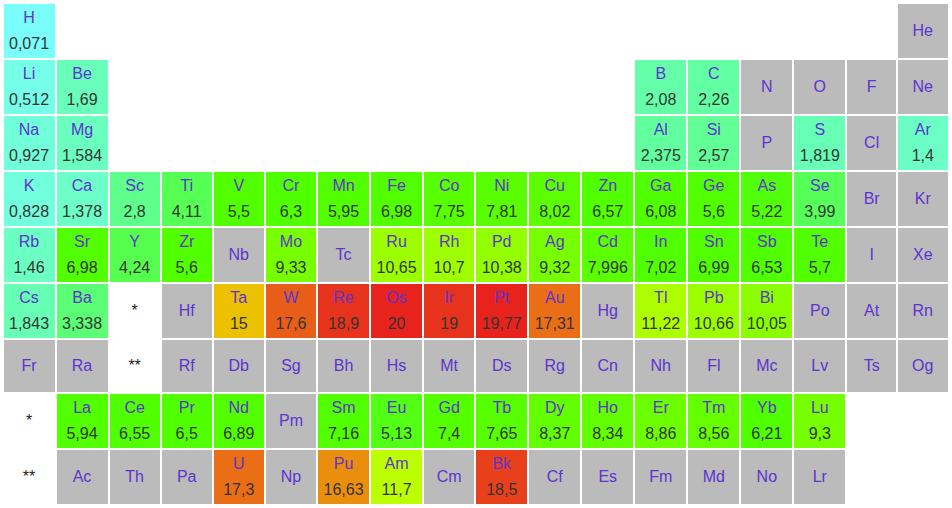
Density of Chemical Elements
Density of chemical elements under laboratory conditions, expressed in (elements with a density greater than osmium or iridium only have a theoretical density: superheavy radioactive elements are produced in quantities too low or decay too rapidly to allow measurement):
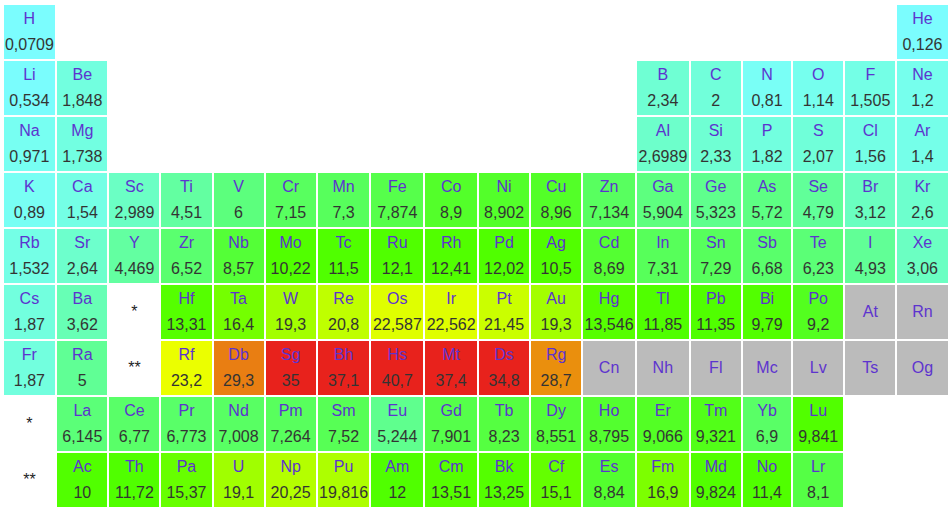
Density of the elements at their melting point in :